The results of various amino acid (AA) supplementations of milk protein-based milk replacers in pre-ruminant calves from Three days to 7 weeks of age have been studied.
Animals have been divided into four teams: Ctrl) Control group fed with milk protein-based milk replacer with out supplementation; GP) supplementation with 0.1% glycine and 0.3% proline; FY) supplementation with 0.2% phenylalanine and 0.2% tyrosine; MKT) supplementation with 0.62% lysine, 0.22% methionine and 0.61% threonine. For statistical analysis, t-test was used to check AA-supplemented animals to the Ctrl group.
At week 7, physique weight and common every day achieve (ADG) have been measured and blood samples and skeletal muscle biopsies have been taken.
Blood biochemistry analytes associated to vitality metabolism have been decided and it was proven that MKT group had larger serum creatinine and larger plasma focus of three supplemented AAs in addition to arginine in contrast with the Ctrl group.
GP group had comparable glycine/proline plasma focus in contrast with the opposite teams whereas in FY group solely plasma phenylalanine focus was larger in contrast with Control.
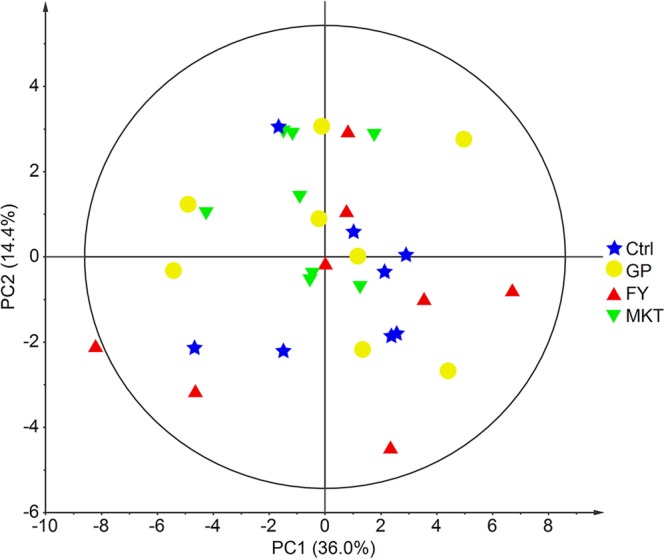
Although the AA supplementations in the GP and FY teams didn’t have an effect on common every day achieve and metabolic well being profile from serum, the metabolome analysis from skeletal muscle biopsy revealed a number of variations between the GP-FY teams and the Ctrl-MKT teams, suggesting a metabolic adaptation particularly in GP and FY teams.
Effects of Nano-Composite Adsorbents on the Growth Performance, Serum Biochemistry, and Organ Weights of Broilers Fed with Aflatoxin-Contaminated Feed.
The exploration of feed mycotoxin adsorbents to mitigate the adversarial results of mycotoxins on animals has acquired rising consideration over the past decade.
The current examine was performed to evaluate the efficacy of nano-composite magnetic graphene oxide with chitosan (MGO-CTS) adsorbents in opposition to feed contaminated with ~20 ng/g (ppb) aflatoxin (AF).
A complete of 300 1-day-old chicks have been randomly distributed into six dietary therapy teams, as follows: basal weight-reduction plan (broilers fed a weight-reduction plan with neither AF nor MGO-CTS added, T1), basal weight-reduction plan + 0.25% MGO-CTS (T2), basal weight-reduction plan + 0.50% MGO-CTS (T3), AF weight-reduction plan + 0.25% MGO-CTS (T4), AF weight-reduction plan + 0.50% MGO-CTS (T5), and AF weight-reduction plan (T6).
The two inclusion ranges (0.25 and 0.50%) of MGO-CTS considerably (p < 0.05) improved the expansion performances and feed conversion ratios of the AF-treated chicks at 1⁻35 days of age, and the influence was extra pronounced for 0.5% MGO-CTS.
The AF consumption markedly elevated the relative weights of the liver and kidney, ensuing in important alterations in the serum biochemical parameters, akin to albumins, alkaline phosphatase, and SGPT/alanine (ALT), at 35 days of age.
However, the chickens fed 0.5% MGO-CTS with AF diets had obvious restoration or restoration of AF-induced organ lesions and aberrant serum profiles. A big (p < 0.05) discount in the entire AFs was noticed in the gastrointestinal tracts of the chickens fed 0.25% or 0.50% adsorbent in mixture with AF feed (T4 and T5), with decreases of 28.9% and 53.5%, respectively, in contrast with that in the chickens fed an AF-contaminated weight-reduction plan (T6).
The outcomes of the examine indicated {that a} larger focus of MGO-CTS (0.50%) was efficient in enhancing the general efficiency of broiler chickens by stopping the adversarial results associated with aflatoxicosis.
