Introduction
Using simple laboratory techniques, demonstrate the production of the enzyme action and the Hydrolysis of invertase (sucrose) by yeast. The demonstration can lead to a discussion of the role invertase plays in yeast metabolism.
Concepts
- Enzymes
- Hydrolysis
Materials (for each demo)
- Qualitative Benedict’s solution, 60 mL
- Filter paper, creped or fast speed
- Sucrose, 5 g
- Filter funnel
- Active dry yeast (bakery), 7 g package
- 10 ml graduated cylinder
- Water, distilled or deionized
- Heating plate
- Beaker, Pyrex, 600 or 1000 mL
- Test Tube Rack
- Beral type pipettes, 3
- Cylinders, 18 × 150 mm, 6
Safety precautions
Benedict’s qualitative solution is irritating to the skin and eyes. Wear chemical splash goggles, chemical resistant gloves, and an apron. Wear insulated gloves or test tube clamps when handling heated test tubes during the Benedict procedure. Please check current Material Safety Data Sheets for additional safety, handling and disposal information.
Preparation
Prepare a yeast filter solution by mixing one packet of active dry yeast with 80 mL of distilled or deionized water. Let stand for approximately 20 minutes with occasional shaking. Filter the resulting suspension and save the filtered solution— this is the crude extract of invertase.
Vacuum filtration or centrifugation are time-saving options to gravity filtration if the equipment is available. Refrigerate the invertase extract if it is kept overnight. Prepare a 5% sucrose solution by dissolving 5 grams of sucrose (highest purity available) in 100 mL of distilled or deionized water. Prepare sucrose solution in brief before using.
Procedure
- Prepare a boiling water bath using a Pyrex beaker and hot plate or Bunsen burner setup (used in step 5).
- Place three test tubes (labelled A1, A2, A3) in a test tube rack. In tube A1, place 20 mL of 5% sucrose solution. Inside tube A2, place 20 mL of 5% sucrose solution and 4 mL of invertase extract. In tube A3, place 20 mL distilled or deionized water and 4 mL of invertase extract. Cap and invert each tube to mix.
- Incubate tubes for 35 minutes in warm water at 30-35°C. Use warm tap water (check temperature) in a beaker or Styrofoam cup.
- Place three more test tubes (labelled B1, B2, B3) in a test tube rack. Place 20 mL of Benedict’s qualitative solution in every transfer 32 drops of the contents of A1 to B1, 32 drops of A2 to B2, and 32 drops of A3 to B3.
- Place tubes B1, B2, and B3 in a boiling (or near boiling) water bath. After three or four minutes, write down whatever the changes are evident.
Provision
Please refer to your current Flinn Scientific catalogue/reference manual for general guidelines and specific procedures governing the disposal of laboratory waste. All resulting mixtures can be flushed down the drain in accordance with Flinn’s recommendation.
Advice
• A “standard” glucose tube can be added in step 4 for comparison. Label this tube B4 and into it place 20 mL of Benedict’s qualitative solution and 32 drops of a 5% glucose (dextrose) solution. Put tube B4 in the boiling water bathroom with the others in step 5.
• Standard proportions for Benedict’s test (qualitative) are 5 ml of Benedict’s solution and 8 drops of sugar/test solution. The volumes in this lab are scaled for visibility.
Discussion
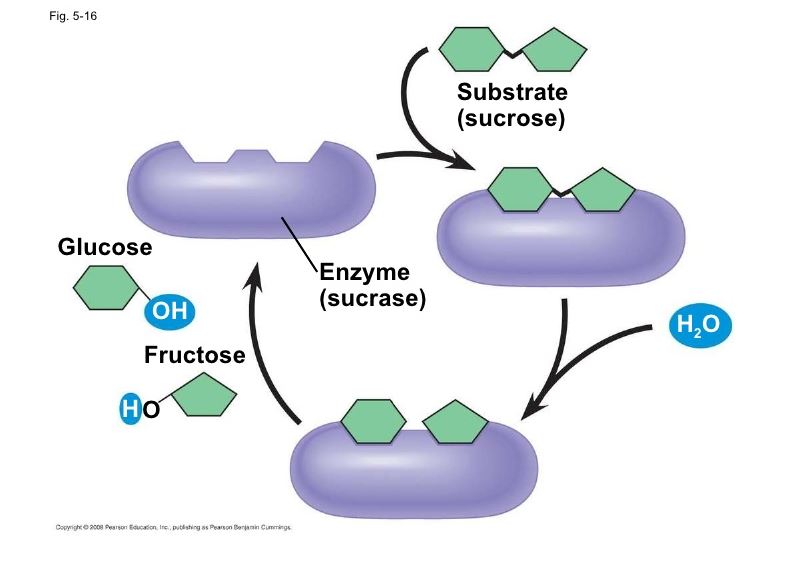
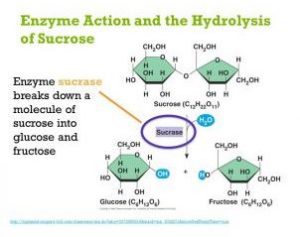
The enzyme invertase (sucrase, sucrase) catalyzes the hydrolysis of sucrose (a disaccharide) to convert it to invert sugar. Invert sugar is a 50/50 mixture of glucose and fructose, both monosaccharides. Yeast cannot directly metabolize (ferment) sucrose. With the purpose of sucrose as an energy source, the yeast must first convert it into fermentable monosaccharides: glucose and fructose. Benedict’s solution is used to show whether or not the conversion of sucrose to invert sugar has occurred.
Benedict the qualitative solution is a test reagent that reacts positively with reducing (simple) sugars. All monosaccharides and most disaccharides are reducing sugars, that is, they have a free or potentially free carbonyl group (C = O). Sucrose is an exception in that it is not a reducing sugar. A positive Benedict’s test is evidenced by the formation of a brownish-red cuprous layer oxide precipitate Both glucose and fructose give positive results with Benedict’s solution, but sucrose does not.
Tubes A1/B1 show that sucrose alone will not reduce Benedict’s solution. Tubes A2/B2 clearly show the capacity of reducing Benedict’s solution, from which we can infer the presence of reducing sugars (in this case, glucose and fructose). Tubes A3/B3 serve as an experimental control to show that the positive reaction is not due to the invertase extract itself. If a glucose standard tube (B4) A1/B1 is incorporated, can be compared directly with tube B2 following Benedict’s test